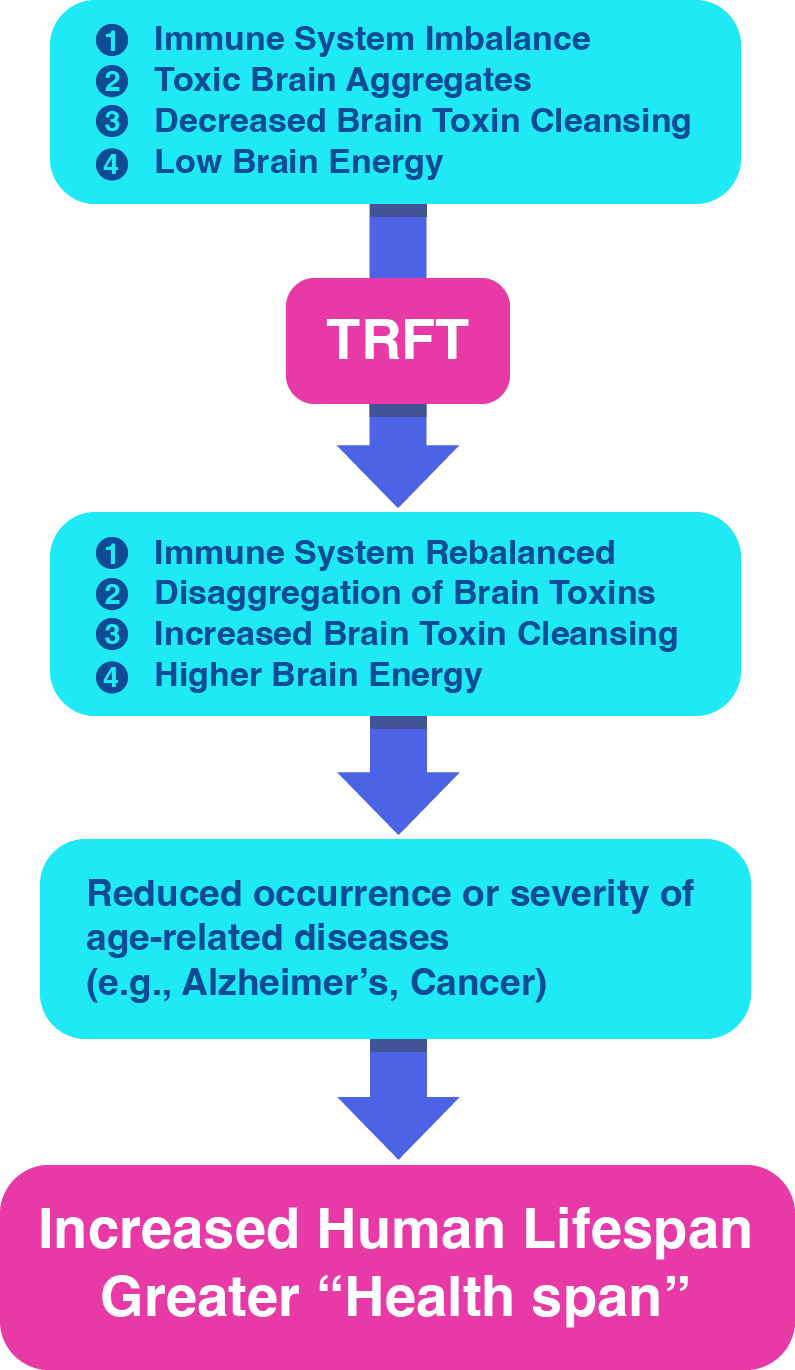
TRFT’s Multiple Mechanisms of Action
TRFT has four “disease-modifying” mechanisms of action that target many diseases of aging, and normal aging itself, to likely increase human longevity.
-
TRFT re-balances the body and brain’s immune system
-
TRFT disaggregates toxic proteins in the brain
-
TRFT increases brain toxin cleansing / drainage
-
TRFT Increases energy production in the brain
TRFT re-balances the body and brain’s immune system
TRFT disaggregates toxic proteins in the brain
TRFT increases brain toxin cleansing / drainage
TRFT Increases energy production in the brain
Mechanism 1:
TRFT Re-balances the Body and Brain’s Immune System
Question: What do all of the below diseases of aging have in common?
- Alzheimer’s Disease
- Brain Cancer
- Multiple Sclerosis
- Chronic Kidney Disease
- Arterial Hypertension
- Parkinson’s Disease
- Cardiovascular Disease
- Bacterial/Viral Infections
- FTL Dementia
- Body Cancers (e.g., lung)
- Amyotophic Lat Sclerosis
- IBD
- Diabetes
- Rheumatoid Arthritis
- Lymphomas
- Peripheral Neuropathy
- Chronic Pain
- LATE Dementia
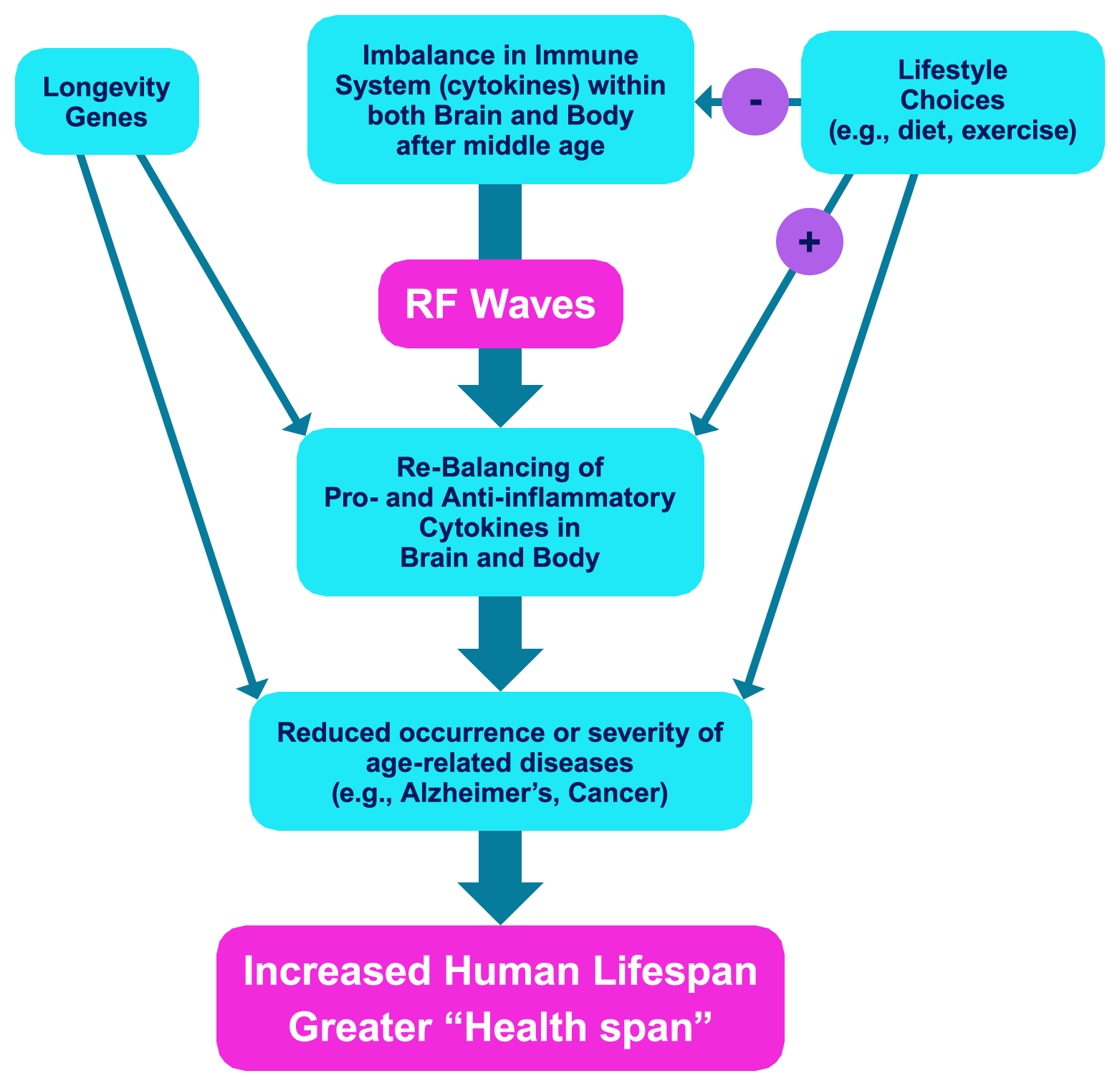
Answer: An imbalanced immune system in later years, also known as “Inflamm-aging”. This imbalance is due to over-activation of the immune system’s pro-inflammatory component over its anti-inflammatory component, as indexed by the immune system’s effectors — “cytokines”.
Another Question: Aside from possible genetic advantages, what is it about Centenarians that provides for their extreme longevity?
Answer: They maintain a balanced immune system.

In young adulthood and through middle age, the immune system’s pro-inflammatory and anti-inflammatory cytokines are in balance.

This cytokine balance is lost in older age, because of over- activation of pro-inflammatory cytokines. This imbalance induces a chronic level of inflammation in the brain and body – the primary cause of many diseases of aging.

Many Centenarians have maintained a “balanced” immune system, with both robust pro-inflammatory and anti-inflammatory components, to effectively provide them with healthy longevity.
Since both pro- and anti-inflammatory cytokine components stay strong through the lives of centenarians, an intervention that can maintain or re-establish immune system balance, wherein both pro- and anti-inflammatory components stay strong, will provide the immune profile seen in centenarians—thus resulting in an increase in lifespan with fewer age-related diseases occurring, as enjoyed by centenarians. There is only one such clinical intervention that has the proven capacity to “rebalance” the human immune system − TRFT.
In a clinical trial involving AD subjects who received 2-months of daily TRFT, blood and brain/CSF levels of twelve cytokines were evaluated at baseline and at 2-months [Cao and Arendash, 2022]. For eleven of the 12 cytokines, blood levels were re-balanced − if baseline levels were below normal, TRFT increased their levels to near normal, and if baseline levels were above normal at baseline, TRFT decreased those levels to near normal. Two examples of this re-balancing in blood for cytokines IL-17α and IL-18 are shown in the figure.

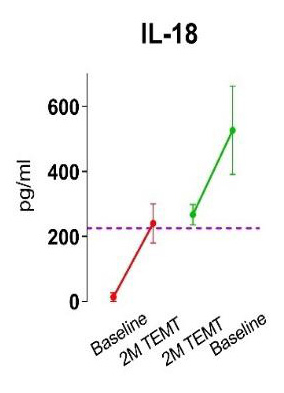

Such re-balancing of the immune system in blood resulted in dramatic decreases in blood/body inflammation in AD subjects. As indexed by blood C-Reactive Protein (CRP), this large decrease in inflammation is evident even at 1-2 years into TRFT (Figure, right) [Arendash et al., 2022]. TRFT is able to affect “blood” cytokine levels because it affects the red blood cells going through the brain’s blood vessels. A very similar re-balancing effect of TRFT on all seven “brain” cytokines measured is also present in AD subjects, as is a resultant striking decrease in brain inflammation [Arendash et al., 2022]. Thus, TRFT affects both the body and the brain immune components to likely increase human longevity:
Mechanism 2:
TRFT Disaggregates Toxic Protein Oligomers in the Brain
Although neuritic plaques and neurofibrillary tangles in the brain have long been thought to be the cause of AD, this is no longer the case in view of compelling newer evidence that toxic protein aggregates (oligomers) inside neurons are the real culprits [Arendash 2016; Arendash et al., 2022]. More specifically, small toxic oligomers of two proteins (Aβ and p-Tau) increase in neurons during aging and much more so in AD (see below figure, left). In AD, these oligomers cause neurons to dysfunction (e.g., mitochondrial and microtubule dysfunction), which ultimately leads to their death. Drugs have thus far failed to prevent, stop, or reverse AD in large part because they have great difficulty getting inside neurons and because they do not specifically target Aβ and p-Tau oligomers.
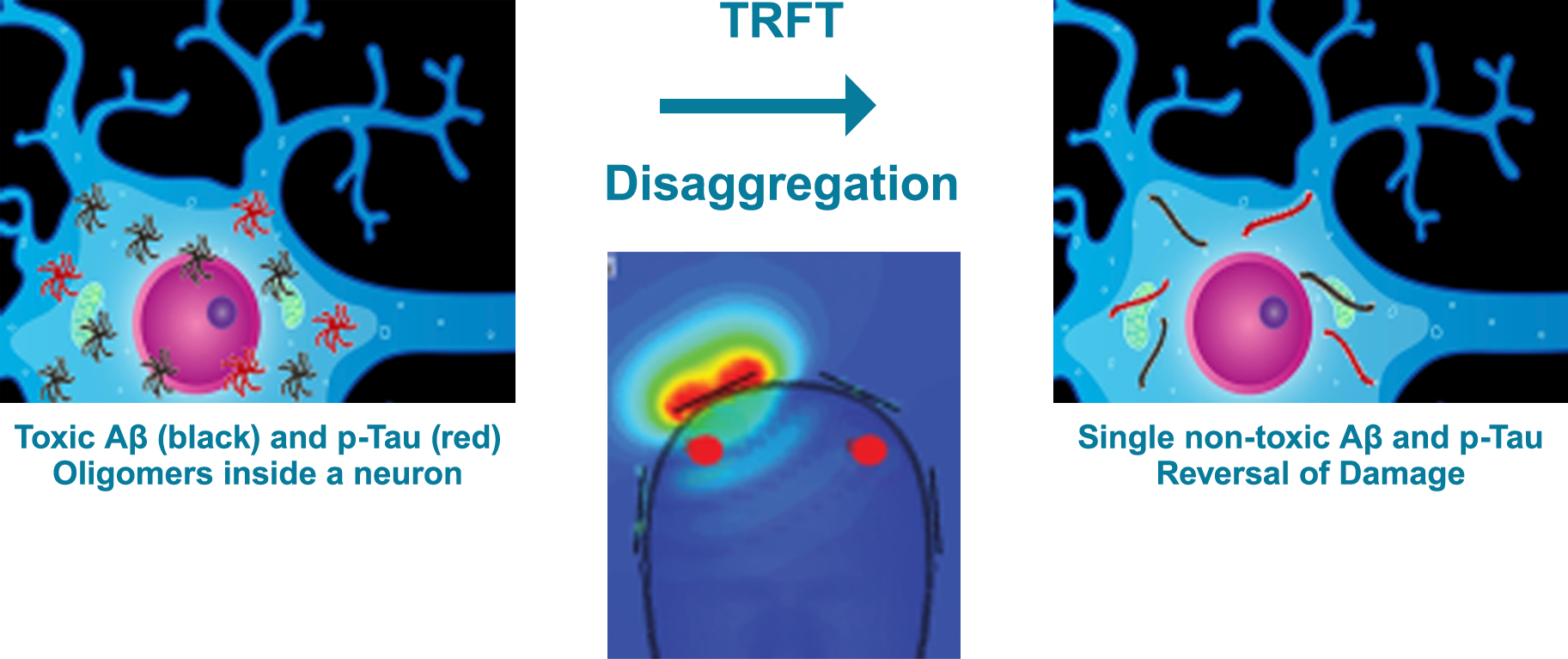
In sharp contrast, TRFT disaggregates BOTH of these toxic oligomers inside neurons into single (monomeric) proteins that are non-toxic (see above figure, right). This results in a substantial decrease in these two toxic oligomers and ensuing reversal of the damage they cause, including reversal of cognitive impairment in AD subjects [Arendash et al., 2019].
Evidence for TRFT-induced disaggregation of Aβ oligomers has been attained from homogenized brains from AD subjects [NIH grant R43NS090653-01A1, Arendash/NeuroEM Therapeutics], wherein a sharp decrease in oligomeric Aβ in frontal cortex is seen within a few days of TRFT. In these same human brain homogenates, (oligomerized) p-Tau is decreased substantially within a few days of TRFT. Additional evidence for TRFT’s ability to disaggregate p-Tau oligomers is clinically present in living AD subjects (see “TRFT Clinical Studies” tab).
In addition to Alzheimer’s Disease, there are a host of age-related neurologic conditions whose pathogenesis involves toxic protein oligomers (Aβ, p-Tau, α-synuclein, and others) that are targeted by TRFT (see below listing) − this, because they all have the same basic structure that is vulnerable to disaggregation by specific radiofrequency waves [Arendash et al., 2019; 2022]:
- Mild Cog. Impairment
- Parkinson’s Disease
- Amyotrophic Lat Sclerosis
- Huntington’s Disease
- Traumatic Brain Injury
- FTL Dementia
- LATE Dementia
- Lewy Body Disease
- Cortico-Basal Dementia
Mechanism 3:
TRFT Induces Brain Toxin Cleansing
While drainage/removal of fluid and toxins (e.g., Aβ, tau) from the brain by CSF flow directly into venous blood is well-known, a second drainage route has recently been (re)discovered − meningeal lymphatic vessels (mLVs). These vessels (shown as green in diagram below) are responsible for up to half of total toxin removal from the brain by transporting brain CSF to cervical lymph nodes, then into the venous circulation (see figure). In our recently published study [Arendash et al., 2024], blood and brain levels of Aβ and tau toxins were measured at several months and at 1-2 years into TRFT. At both time points, TRFT was shown to increase the “brain cleansing” (removal) of Aβ and tau from the brain and into plasma of AD patients. This appears to be due to TRFT’s ability to enhance CSF outflow by increasing mLV flow, which was likely contributory to TRFT’s cognitive benefits in the same AD subjects. Thus, TRFT provides both toxin disaggregation in the brain (Mechanism 2) and their subsequent removal from the brain (Mechanism 3).
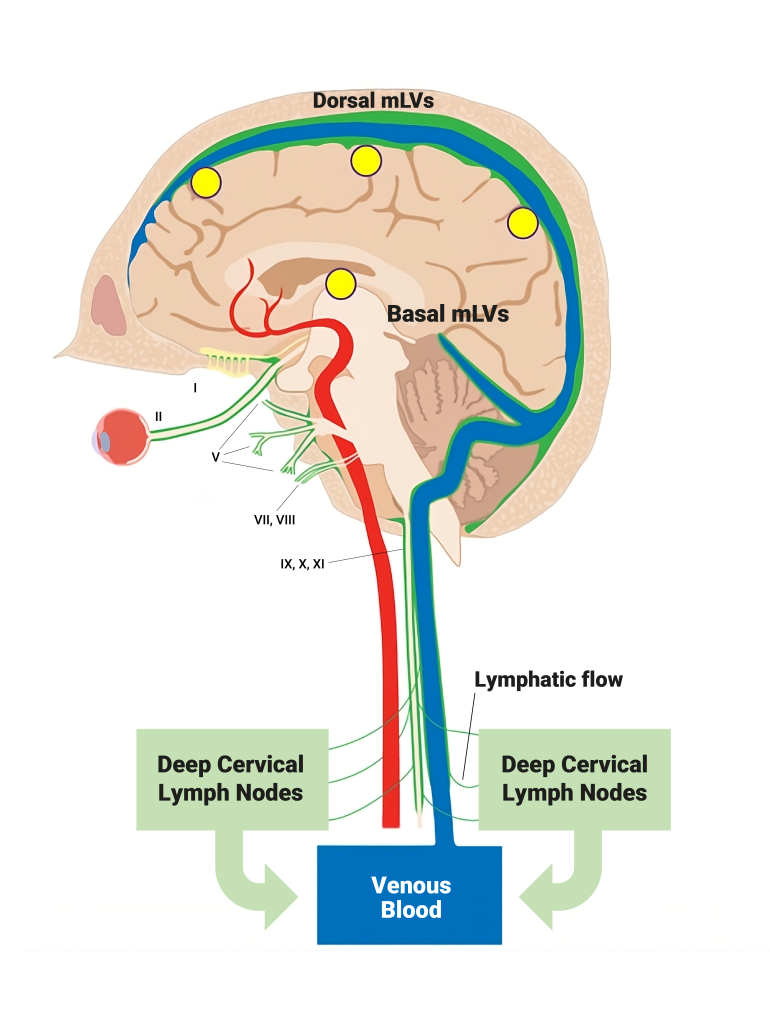
It is important to recognize that almost all brain cleansing of toxins and metabolic wastes normally occurs during sleep. Yet remarkably the AD patients received daily TRFT during mid-morning hours (i.e., during wakefulness). As such, TRFT performs a major duty of sleep during wakefulness, which was not initially recognized in Arendash et al. 2024, but later revealed in Arendash [2025] a few months thereafter. This new TRFT mechanism is important because of the reduced sleep and/or sleep disorders seen during normal aging and disease. Thus, TRFT during wakefulness is the first clinical intervention to counter the decline in nighttime brain toxin cleansing that occurs with normal aging and in multiple brain diseases (most notably Alzheimer’s Disease). Since these brain cleansing studies involved only a small number of subjects, it is essential to conduct additional TRFT clinical trials in both normal aged humans and those with neurologic disorders of aging to determine TRFT’s true potential for human brain cleansing.
In a broader perspective, TRFT has the potential to counter deleterious effects of insomnia and sleep deprivation on brain toxin cleansing in otherwise normal individuals and to even reduce the amount of sleep normally required for brain toxin cleansing.
Mechanism 4:
TRFT Increases Energy Production in the Brain
Initial indications that TRFT is a mitochondrial/metabolic enhancer to increase the brain’s energy production were attained from our pre-clinical studies wherein both “aged” AD transgenic mice and normal mice were given one month of daily RF wave treatment [Dragicevic et al., 2011]. In aged AD mice, RF wave treatment enhanced brain mitochondrial function by 50-150%, especially in cognitively-important brain areas. Even normal “aged” mice showed an increase in mitochondrial function. Moreover, some human AD subjects given 2 months of daily TRFT showed a substantial increase in cerebral metabolic rate, as indicated by FDG-PET scans taken before and after 2 months of treatment (see figure) [Arendash et al., 2019]. Therefore, TRFT appears to be a brain metabolic/energy enhancer in humans.

SUMMARY:
Four Mechanisms of TRFT Act to Likely Extend Human Health Span