A Major Obstacle Against Extending Human Longevity is Alzheimer’s Disease: The TRFT Solution
Among the leading diseases of aging that shorten human life span is Alzheimer’s Disease (AD). This progressive neurodegenerative disease robs individuals of their memory over a 2-10 year period. One in three Americans dies with AD, while seven million currently have AD and another six million have the prelude to AD called Mild Cognitive Impairment (MCI). For several decades now, researchers have been searching for a therapeutic to prevent or treat AD. Unfortunately, there remains no drug that can prevent, stop/stabilize, or reverse AD’s cognitive decline.
Therefore, any gerotherapeutic intervention with hopes of significantly extending human health span should successfully prevent, stop, and/or reverse AD. That intervention must target the AD pathogenic process itself, which occurs within the brain’s neurons. Based on clinical results, safety, and mechanisms of action, that intervention is TRFT.
Summarized below are published clinical finding that describe the exciting cognitive, AD marker, and brain imaging effects of TRFT technology. Then the multiple disease-modifying mechanisms of TRFT that Dr. Arendash has shown to stop and reverse AD cognitive decline are described under the “TRFT Mechanisms” tab. Note: Dr. Arendash’s extensive pre-clinical studies are listed as the lower six publications under “Publications” tab.
Published Clinical TRFT Studies
Dr. Arendash’s clinical work into longevity has primarily targeted one major disease of aging (Alzheimer’s Disease), but through multiple mechanisms that should successfully prevent/lessen many diseases of aging. A brief description of this published clinical work, which involved numerous collaborators (see “Publications” tab, top seven publications), is presented below:
TRFT is the only AD therapeutic that has been shown to reverse AD cognitive decline in clinical trials (Phase IIa), although in a limited number of subjects. [Arendash et al., 2019]. TRFT was administered to AD subjects daily in-home for 2-months. Both ADAS-cog13 and Rey AVLT performance were dramatically improved after 2 months, and to the better cognition these subjects would have had one year earlier. This cognitive benefit was present even two weeks after TRFT stopped.
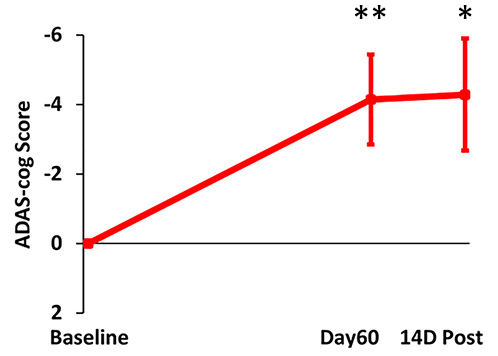
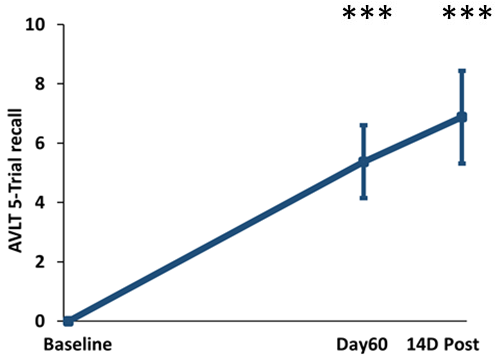
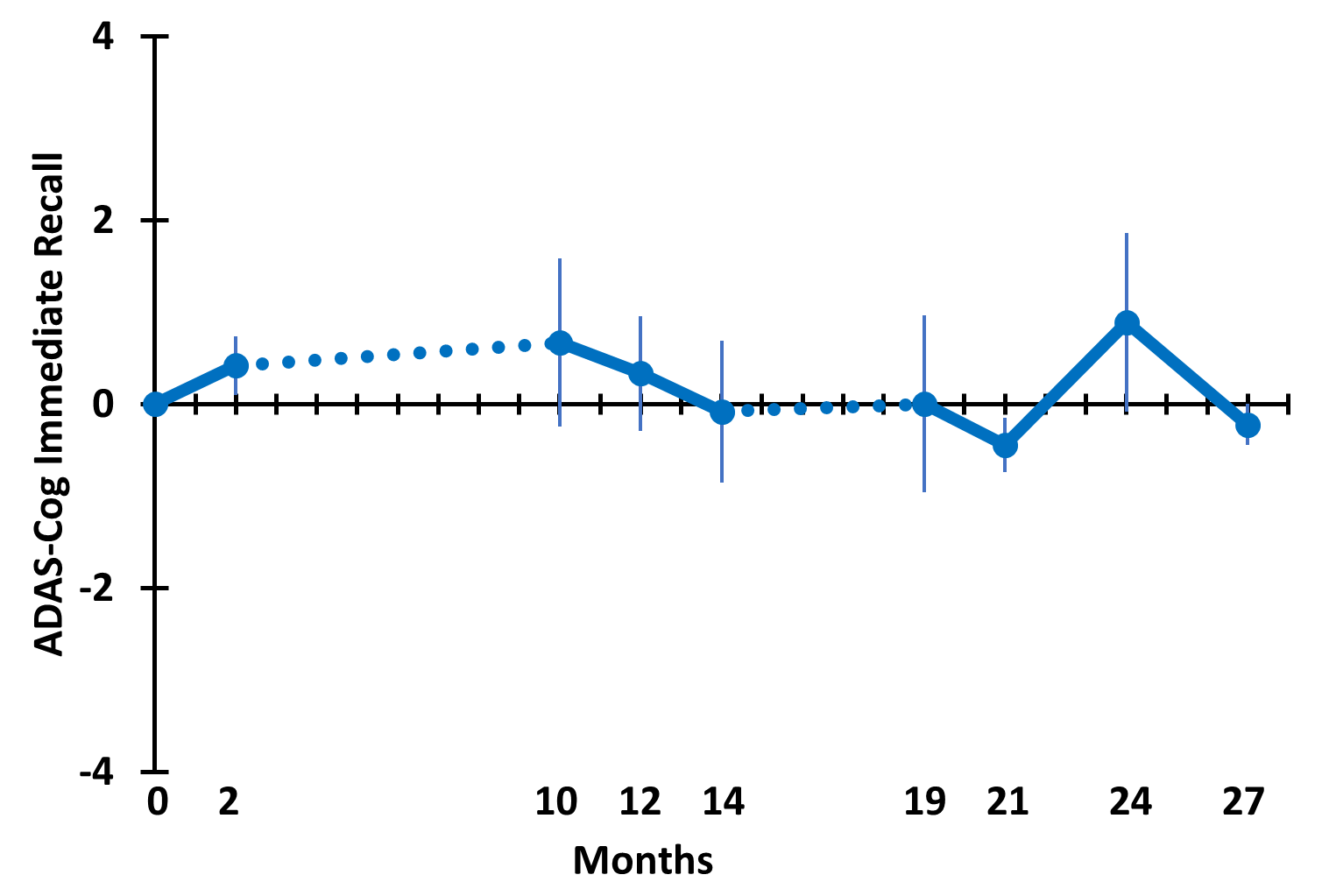
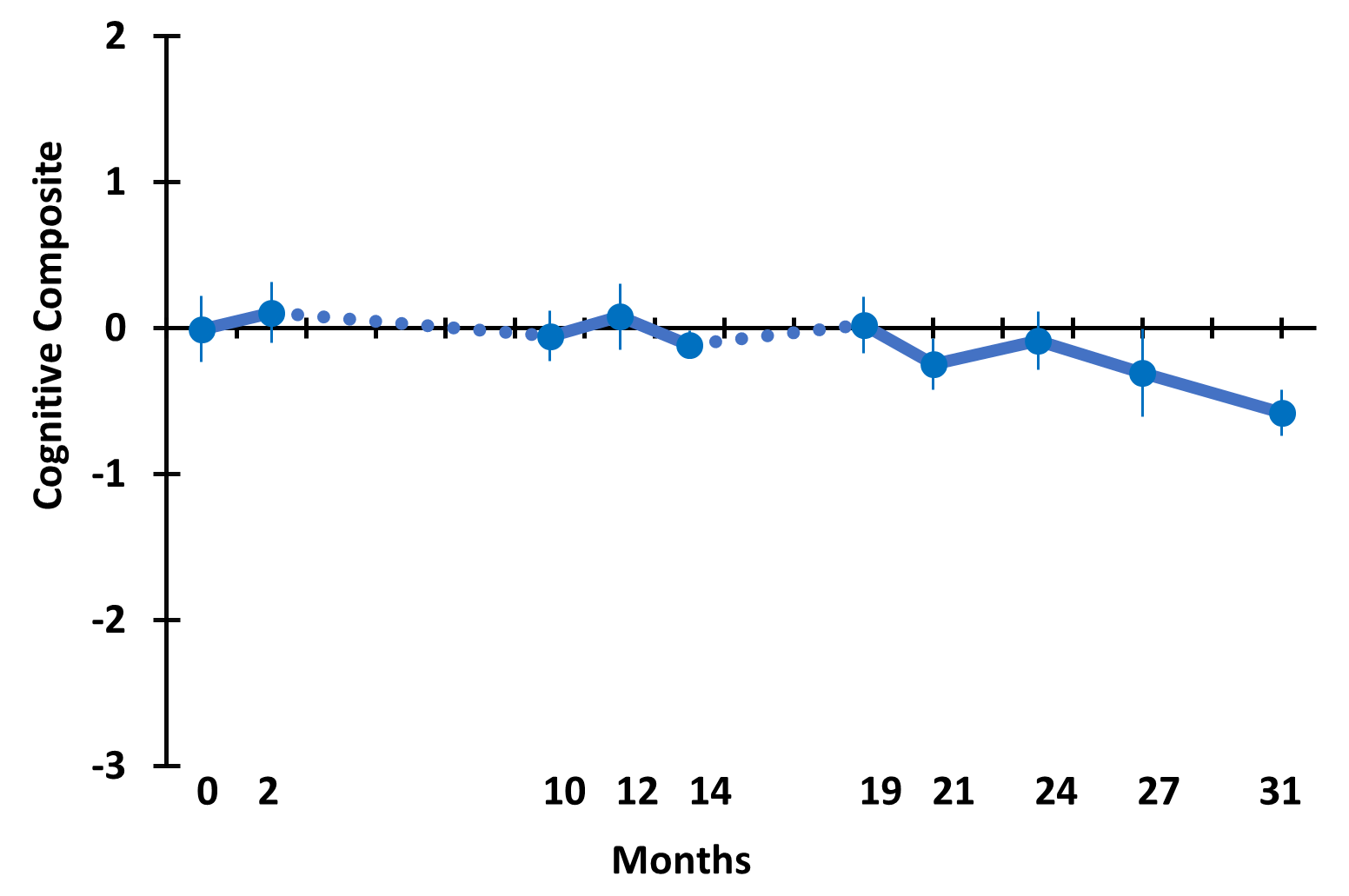
TRFT is the only AD therapeutic that has been shown to stop the progressive cognitive decline in AD subjects over a long, protracted period (2½ years), though in a small study [Arendash et al., 2022]. TRFT was administered daily for 18 months over a 31 month period (dashed lines in below graphs indicate the two periods of no treatment). ADAS-cog IR is shown (below left), while a “Cognitive Composite” graph (below right) shows “overall cognitive performance” combining eight cognitive measures from six tasks, including ADAS-cog13. No cognitive decline occurred over the 2½ year period for any of the eight measures individually or collectively, indicating TRFT had stopped the progression of cognitive loss over a protracted period. Note maintenance of cognitive performance even after 8- and 5-month periods of no treatment.
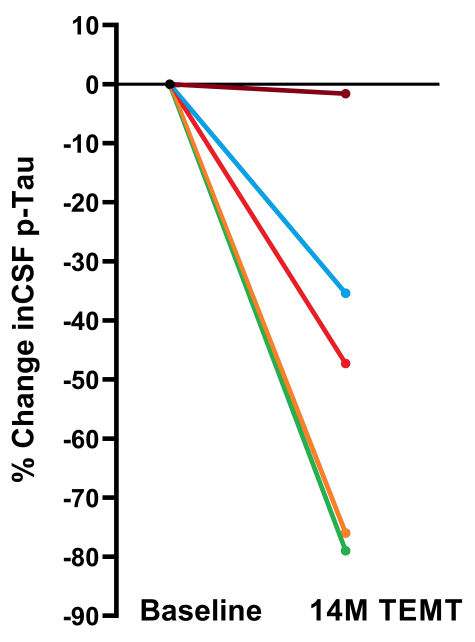
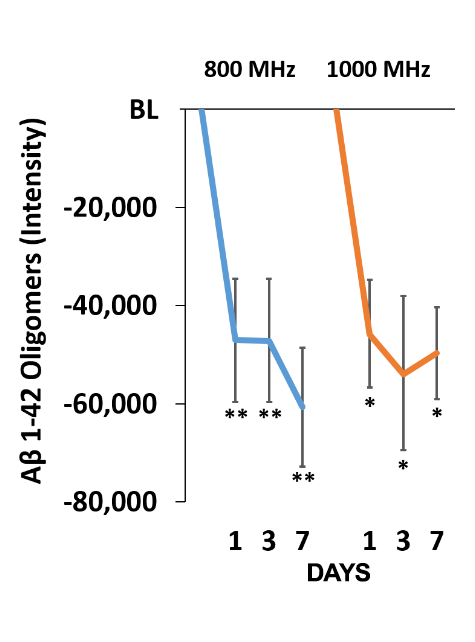
TRFT beneficially decreases major AD markers in brain/CSF, such as p-tau and Aβ oligomers [Arendash et al., 2022]. Shown below (left) is the dramatic decrease in brain/CSF levels of (oligomeric) p-Tau induced by TRFT at 14 months into treatment – CSF p-Tau levels typically increase over time in AD subjects. Below (right) shows the sharp decrease in oligomeric Aβ seen in AD homogenized brains even at one day into daily TRFT and continuing through seven days of treatment.
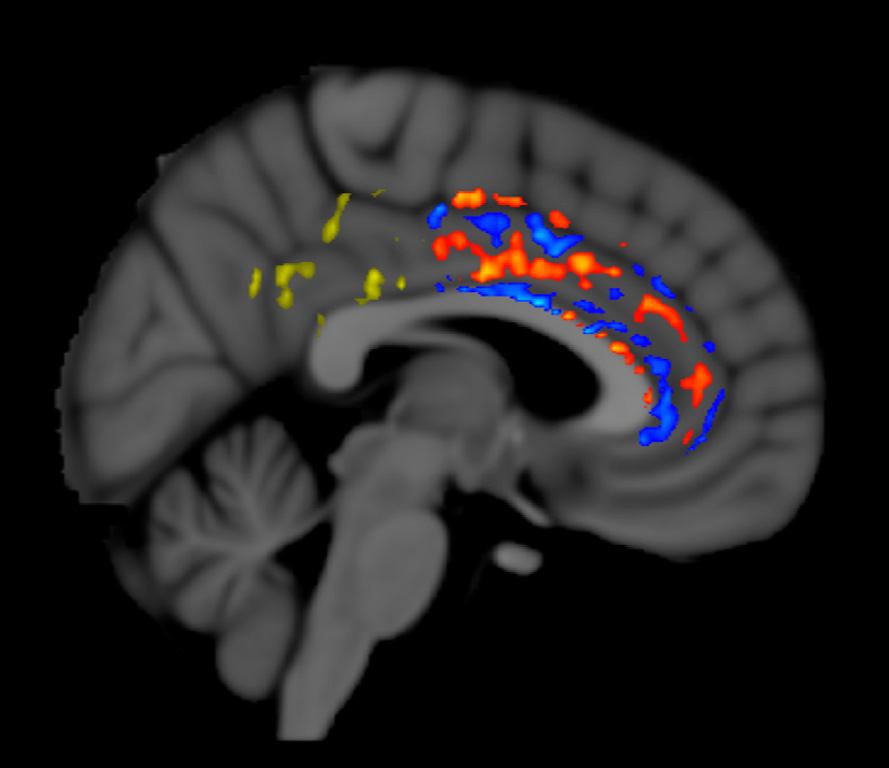
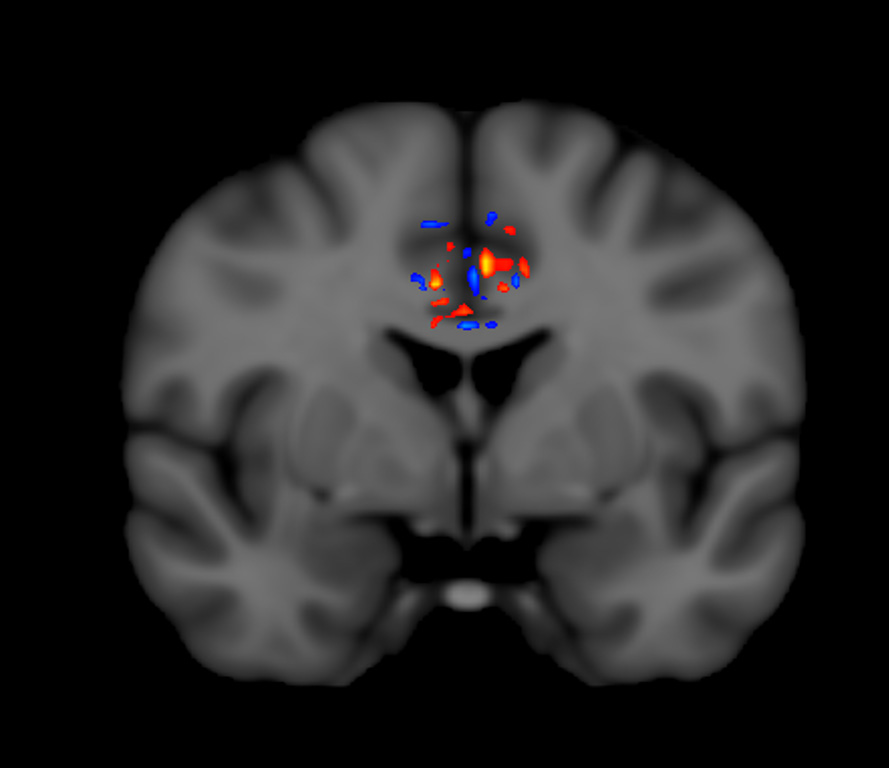
TRFT increases “functional connectivity” of neurons within the brains of all AD subjects evaluated by functional MRI (fMRI). For example, fMRI brain imaging at 2-months into TRFT for an AD subject is shown below in an important brain area for cognitive integration, the Cingulate Cortex. “Functional connectivity” within the AD brain typically decreases over time in fMRI imaging and indeed there were such areas of decreased fMRI signaling seen (blue pixels). However, there were also many areas of increased fMRI signaling (orange, yellow pixels) in this and all other AD subjects − something that is never seen over time in the brains of untreated AD subjects. Thus, TRFT actually increased functional connectivity (communication of neurons with one another) in an important cognitive brain area. This fMRI enhancement could be involved in the reversal of cognitive impairments seen in the same AD subjects [Arendash et al., 2019].
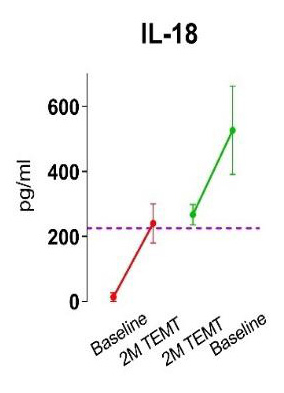
TRFT re-balances the immune system (cytokines) in both brain and body to dramatically decrease brain and body inflammation. Inflammation is central to most diseases of aging, so TRFT should target those diseases (see “TRFT Mechanisms” tab)


What’s Needed Now
THE FIRST THERAPEUTIC LONGEVITY TRIALS IN HUMANS. Longitudinal 5-month and 5-year Phase IIb/III controlled clinical trials with TRFT in normal aged individuals to monitor occurrence of many diseases of ageing, biologic age, cognition, and inflammation. These clinical trials would determine if TRFT can reduced the occurrence/severity of those diseases, reverse biologic age, improve cognition, decrease inflammation, and extend health span.